A vast amount of research shows that glyphosate pesticides and other toxins, which are ubiquitous in our environment, impact our fragile but powerful thyroid gland and can increase the risk of hypothyroidism, even beginning in the womb.
Because so many pesticides work on the nervous system, scientists are increasingly discovering the pesticide-related hypothyroidism risks come in part from the impact on the brain’s communication with the thyroid.
What you’ll learn:
- One of the primary ways pesticides appear to impact thyroid function is by disrupting communication between the brain, the pituitary gland, and the thyroid gland. This is called the hypothalamus-pituitary-thyroid (HPT) axis.
- Although organochlorine pesticides (OCPs) like DDT have been banned, they still persist in the environment and impact thyroid health, especially in pregnant women and children. They can also worsen the impact of pesticides currently in use.
- Glyphosate is most commonly associated with the pesticide Roundup weed killer and is linked with an increased risk of hypothyroidism.
- Carbamates are widely used in agriculture as insecticides, herbicides, and fungicides and have been linked to lower thyroid function through their impacts on thyroid enzymes.
- Pyrethroids are synthetic versions of the natural pest deterrent pyrethrin and are abundant in the environment due to their wide use globally. One of their mechanisms is to compete with thyroid hormones at the receptor site.
- Phenylpyrazole is primarily found in the product ingredient fipronil, which is widely used in urban and veterinary applications for pest control. It inhibits thyroid function.
- Neonicotinoids are insecticides that were discovered in the 1980s and are widely used in agricultural, commercial, residential, and veterinary settings. They have been shown to disrupt thyroid function and decrease thyroid hormones, not to mention contribute to the collapse of the bee population.
Synthetic pesticides started to become widely used in the 1940s, and although they significantly increased crop yields (1) (2) (3), they also showed alarming effects on health. While those warnings were ignored over the years as the number of pesticides introduced to the market grew, scientists continued to study (4) their effects on human health.
One trend that has consistently emerged is the link between pesticides and their impact on thyroid health and function, contributing to symptoms of hypothyroidism (low thyroid function) and other thyroid disorders.
The warning bells first rang in the early 1960s when researchers discovered pesticides thinned the eggshells of wild birds due to impact on the endocrine, or hormonal, system (5) (6). Since then, researchers have been studying the impacts of pesticides on human health (7) (8).
The term “endocrine disrupter” was coined in the early 1990s to describe the way pesticides interfere with (9) (10):
- Thyroid hormones
- Estrogens
- Male hormones like testosterone
- Adrenal hormones like cortisol
While the effects of acute exposures on workers in the pesticide industry have been well researched (11) (12) (13), the impact of lower level but cumulative contamination from everyday exposure is less studied (14). Pesticides are abundant not only in agriculture, but also in urban and suburban environments, schools, lawns, playing fields and golf courses, and perhaps even in your own garden.
Given that even low levels of exposure of many pesticides have been shown to disrupt the endocrine system (15) (16) (17) (18), understanding the risks to your thyroid health is vital. This is especially true during pregnancy and early motherhood when the developing fetus and infant are more vulnerable (19) (20).
How Pesticides Affect Communication Between the Brain and the Thyroid
Every cell in the body uses thyroid hormone, so thyroid activity is necessary for every organ and system. However, the thyroid gland is best known for its role in regulating metabolism, which is why thyroid disorders often manifest as weight gain, weight loss resistance, and feeling cold all the time (21) (22).
In children, healthy thyroid function ensures proper brain development (23); thyroid dysfunction during early pregnancy can impact the child’s IQ later in life (24) (25) (26).
In fact, a 2015 European study estimated the impacts of endocrine disrupting pesticides on childhood brain development disorders costs more than 150 billion Euros, or $162 billion annually due to low IQ, autism spectrum disorders, attention deficit/hyperactivity disorder (ADHD), and other issues in children (27).
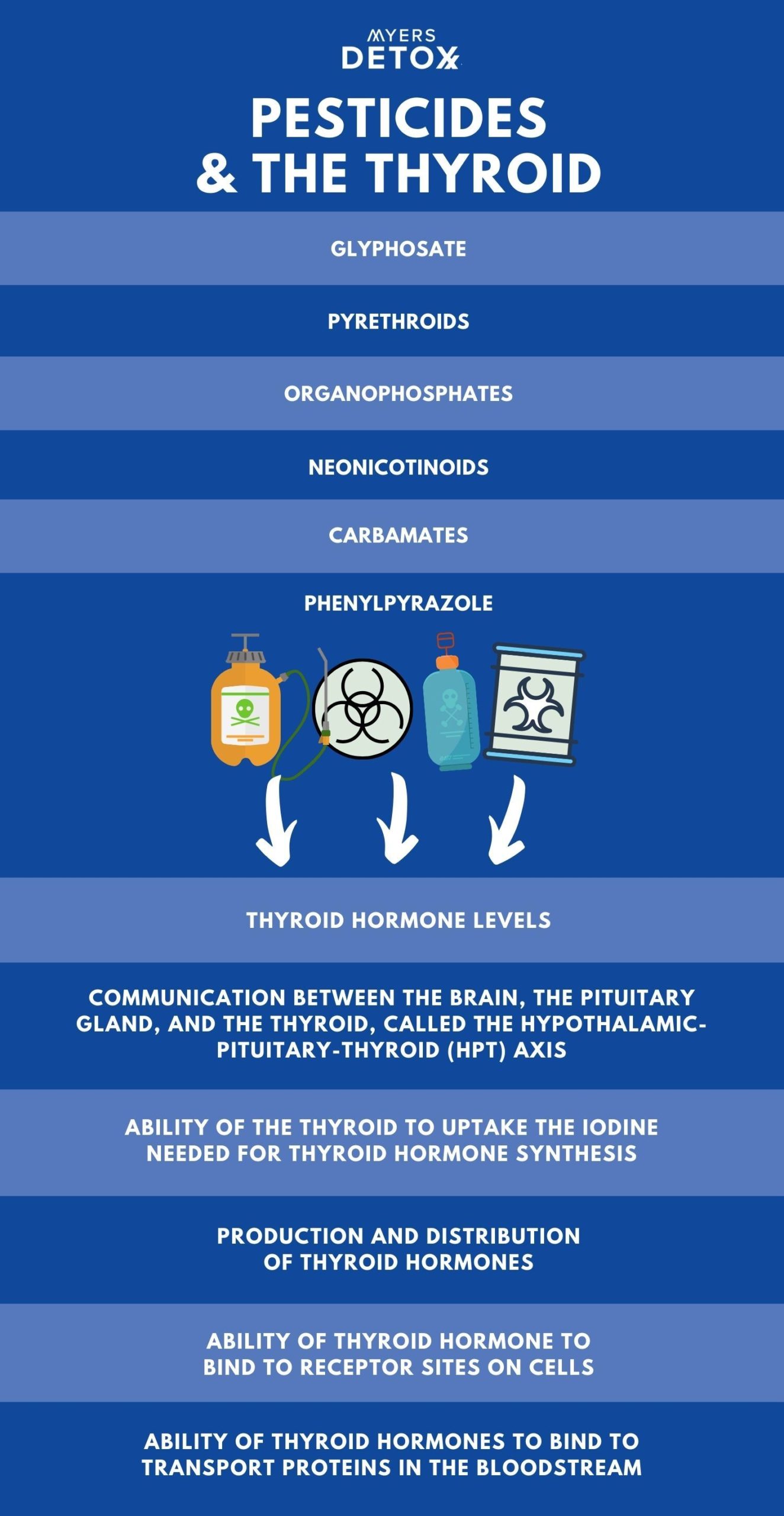
Studies show pesticides impact the following areas of thyroid function (28) (29) (30) (31) (32) (33):
- Thyroid hormone levels
- Communication between the brain, the pituitary gland, and the thyroid, called the hypothalamic-pituitary-thyroid (HPT) axis
- Ability of the thyroid to uptake the iodine needed for thyroid hormone synthesis
- Production and distribution of thyroid hormones
- Ability of thyroid hormone to bind to receptor sites on cells
- Ability of thyroid hormones to bind to transport proteins in the bloodstream
Following is a more detailed breakdown of how different classes of pesticides like glyphosate affect thyroid function.
How Organochlorine Pesticides (OCPs) Damage the Thyroid
Studies on the effects of organochlorine pesticides (OCPs) like DDT on mothers and their children have found associations with either an increase in thyroid-stimulating hormone (TSH), which regulates thyroid activity, a decrease in circulating thyroid hormones throughout the body, or both.
Even though most OCPs have been banned, they persist in the environment and in human tissues, and may exacerbate or potentiate the harmful effects of newer pesticides commonly used today.
OCPs were the first generation of synthetic pesticides and were used extensively globally until the 1960s. They include DDT and the fungicide hexachlorobenzene. OCPs work by disrupting the nervous systems in insects (34).
Effective and cheap, DDT was once widely used around the world (35) until its harmful effects on wildlife were discovered (36). Public outcry over DDT eventually played a role in the creation of the Environmental Protection Agency and the ban of many OCPs in the 1970s.
Despite the ban, OCPs are still very prevalent in the environment and continue to impact human and animal health. Because they do not easily break down, they accumulate in body tissue, persist in the soil and are still found throughout the food chain (37) (38).
OCPs are associated with poor brain development in infants and children (39) (40) (41) (42) (43) (43) (44) (45) (46) (47) (48), which is believed to stem from the impact of OCPs on the hypothalamic-pituitary-thyroid axis (HPT) between the brain and the thyroid in pregnant women and newborns. (49) (50) (51) (52) (53) (54) (55) (56) (57) (58) (59) (60)
The HPT axis is a delicate feedback loop that signals to the glands how much thyroid hormone to produce. The hypothalamus is the area of the brain that governs hormone function. The pituitary gland, located at the base of the brain, secretes hormones that regulate the body’s other hormone glands.
A number of studies on pregnant mothers and children have shown OCPs lower thyroid hormones and thyroid hormone activity in both mothers and newborns. The studies also showed OCPs elevate thyroid-stimulating hormone (TSH).
High TSH is a primary marker for a hypothyroidism diagnosis (low thyroid). When the thyroid is under functioning, TSH goes up in an attempt to tell the brain to raise thyroid hormone levels.
One interesting exception was among a fishing population in Canada (61) that did not show similar impacts. The study’s authors considered the higher intake of dietary iodine and selenium may have buffered the thyroid from the damaging effects of OCPs.
Most people are deficient in iodine and selenium. I always recommend clients to take these two minerals as they are so important for proper thyroid functioning.
Gender also affects outcomes. A 2015 study of the French Caribbean where OCPs were used until 1993 showed that prenatal exposure to OCPs raised TSH in boys (remember, higher TSH means lower thyroid function).
However, in female infants, postnatal exposure was associated with lower thyroxine (T4), the primary thyroid hormone secreted by the thyroid gland, and lower triiodothyronine T3 in both girls and boys (62). T3 is the active form of thyroid hormone that the body’s cells can use.
Studies on the impacts of OCPs on adults are less straightforward. Some show they are linked to higher rates of hyperthyroidism (like in Grave’s disease, an autoimmune disease that causes the thyroid to be overactive) in men (61), aging women (62), and occupational workers (63).
Other studies show OCPs related to hypothyroidism in women (64), elderly men (65), adolescents (66) and pesticide workers that apply pesticides (67) (68) (69) (70) (71).
How are OCPs inhibiting thyroid function specifically? Animal studies suggest that OCPs:
- Impact the proteins that transport thyroid hormones in the blood,
- Increase the clearance of thyroid hormone in the liver,
- Impact receptor sites for thyroid hormone in the brain, and
- Mimic thyroid hormones and competing with them at cellular receptor sites (72) (73) (74) (75) (76) (77).
Long term exposure to low levels of DDT has been shown to change the structure of thyroid tissue to compensate for lowered thyroid hormone activity (78) (79).
In vitro (test tube or petri dish) studies suggest DDT alters cell membranes to cause receptors for TSH to fail (80) (81) (82), negatively impact thyroid hormone production (83), and dysregulate thyroid specific gene function (84).
In summary, studies on OCPs have identified numerous ways in which they inhibit thyroid function and cause symptoms of hypothyroidism or hyperthyroidism.
How Organophosphate Pesticides Disrupt Thyroid Function
Organophosphate pesticides (OPPs) are another type of pesticide, the first application having been as nerve gas in World War II. Because OPPs work similarly on the insect nervous system as they do on the human’s, they were soon introduced for agricultural use (85).
This is unfortunate because studies soon showed that even low levels affected fetal brain development (86) (87). Although most developed countries have phased out use of OPPs, their agricultural use continues to grow in developing nations worldwide (88).
Organophosphates have been associated with thyroid dysfunction (89), increase of hypothyroidism (90) (91) (92), brain function impairment (93) (94), and an increase in hormone-dependent cancers (95).
Research shows that urinary prenatal levels of OPPs are associated with reduced intellect (96), differences in brain structure (97), and higher TSH levels in children, thus indicating compromise of thyroid function (98).
In adults, exposure to OPPs has been shown to raise the risk of developing breast, thyroid, and ovarian cancers, and lymphoma (99). A study on pregnant women showed urinary levels of OPPs were associated with a negative impact on thyroid function (100).
Animal studies using environmentally relevant (not exaggerated) amounts of OPPs also show evidence of thyroid damage. Pre- and post-natal exposure resulted in maternal hypothyroidism and long-term reduction in the thyroid hormone T4 in offspring (101) (102).
Researchers also noted that manufacturers do not disclose the link between OPPs on poor cerebellum development, which they hypothesized stems from thyroid hormone deficiency in the developing fetus (103).
Fish studies have found OPPs interfere with maturation by disrupting thyroid function, altering genes, and lowering thyroid hormone levels, (104) (105) (106) (107), thus affecting the survival of fish in areas contaminated by OPPs (108).
How Glyphosate Damages the Thyroid
Glyphosate is an organophosphate compound used globally to kill weeds, its use having increased significantly since the mid 1990s not only on crops, but on city parks and playgrounds and on residential yards.
Glyphosate is the most used agriculture chemical ever. The most commonly known glyphosate is Roundup weed killer(109).
Trends in glyphosate herbicide use in the United States and globally, reveals that since 1974, when Roundup was first commercially sold, more than 3.5 billion pounds (or 1.6 billion kilograms) of glyphosate has been used in the U.S.. Compare that to the 18.9 billion pounds (or 8.6 billion kilograms) of glyphosate that has been used around the globe. It’s shocking to think the toll this has taken on human and animal health.
One study of more than 35,000 licensed pesticide applicators in the U.S. showed glyphosate was associated with increased risk of hypothyroidism (110), although these results conflict with other research (111) (112).
However, a rat study of pregnant rats exposed to glyphosate showed their thyroid-related genes behaved differently compared to control rats. They showed lower TSH levels (113), which could result from the changes in gene expression and alterations in the HPT axis. When function of the pituitary, the master hormone gland, is suppressed, this can result in low TSH (114) (115).
Researchers state that more studies are needed on the effects of glyphosate on the thyroid as well as on the gut microbiome, which could also impact genetic and thyroid function.
How Carbamates Reduce Thyroid Function
Carbamates work similarly to organophosphates on the nervous system and are widely used in agriculture as insecticides, herbicides, and fungicides (116). They have been shown to inhibit the thyroid peroxidase (TPO), an enzyme necessary to produce thyroid hormones (117) (118) (119).
While exposure is primarily of concern to farm and industrial workers, carbamates also make their way into the food supply of the average consumer (120). For instance, studies in Taiwan and Italy showed subjects with elevated levels of carbamates and OPPs had a significantly higher risk of developing hypothyroidism than the general population (121) (122).
Rat research has shown that carbamates impact thyroid-releasing hormone and thyroid enzyme activity, thus promoting hypothyroidism (123) (124) (125).
Hamster studies show carbamates impact thyroid development (126), while a study of zebrafish embryos exposed to carbamates showed altered thyroid genes and delayed hatching, increased mortality, and produced skeletal defects (127).
How Pyrethroids Alter Thyroid Hormone Levels
While pyrethrins, which are found in Chrysanthemum flowers, naturally protect against insects (128), they are not used commercially much as they break down quickly. Therefore, manufacturers created a synthetic version called pyrethroids, which act on the nervous system of insects (129).
Pyrethroids are used widely around the world (130), and most people are exposed through foods contaminated with the compound (131). They are found in soil, food, environmental, and human samples (132) (133) (134) (135) (136), most likely due to their excessive use.
Pyrethroids structurally resemble the thyroid hormones T3 and T4, which means they compete with thyroid hormone at cellular receptor sites, thus causing symptoms of deficiency (137).
Human exposure is widespread (138), and prenatal exposure via the placenta can impair normal development by impacting thyroid receptor site function, although more research is needed in this area (139) (140) (141).
Rat studies of pyrethroid exposure shows it alters levels of thyroid hormone (142) (143), impacts thyroid genes in the placenta of pregnant rats, and inhibits growth of the fetus (144).
Both lizard and fish studies showed pyrethroids alter thyroid-related genes (145) (146) and disrupt communication between the pituitary gland and the thyroid via the HPT axis (147).
Another fish study showed they impact the signaling behavior of thyroid hormones and thus development of embryos (148) (149).
How Phenylpyrazole Inhibit Thyroid Hormones
Phenylpyrazole is primarily found in the product ingredient fipronil, which is widely used to kill termites, ants, and roaches. Pets and humans are exposed via flea and tick collars and flea prevention medications like Frontline, Sentry Fiproguard, PetArmor, and Hartz First Defense. (150).
Fipronil’s popularity (151) (152), has led it to become a widespread environmental contaminant found in both soil, water (153) (154), dust (155), and foods (156) (157).
Most European nations restricted fipronil in 2013 after it was found to play a large role in the mass deaths of honeybees (158), although this ban has since been overturned (159).
Unfortunately, while it kills pests, it also kills beneficial insects and adversely affects fish, reptiles, birds, and mammals (160) (161) (162) (163) (164) (165) (166) (167) (168) (169).
In studies of factory workers who manufacture veterinary drugs, fipronil exposure is associated with inhibited secretion of TSH (170). Studies on the general population show fipronil transfers to the fetus through the placenta and is correlated with lower levels of T3 in newborns (171) and poor Apgar scores, a method used to assess newborns after birth (172).
In rat studies, fipronil exposure caused tumors via excess growth of thyroid follicles (173), disrupted the development of thyroid tissue (174) (175), and decreased levels of circulating thyroid hormone levels (176) (177) (178) (179).
How Neonicotinoids Decrease Thyroid Function
Neonicotinoids are insecticides that were discovered in the 1980s and are widely used in agriculture (180), and commercial, residential, and veterinary settings (181). In fact, only glyphosates are more popular (182) (183). Neonicotinoids are banned in Europe for killing bees and other pollinators.
As the name suggests, they are structurally similar to nicotine, which was once used as an insecticide, and disrupts the nervous system of insects (184) (185).
One study shows a connection between prenatal exposure to neonicotinoids and reduced IQ (186).
Other research shows exposure disrupts thyroid function in male finches (187), with excessive growth of the thyroid gland, and decreases in thyroid hormones.
How the Interaction of Pesticides Worsen Thyroid Function
Although scientists have studied some of the tens of thousands of chemicals in our environment, it’s important to consider how toxins affect our health when combined, as pesticides often are for agricultural use (188).
Chemicals can interfere or interact with one another (189), and health impacts can manifest when chemicals are combined that were not found when they were studied in isolation (190) (191) (192). These chemicals can potentiate each other, making them more toxic than when exposed to a single chemical.
For instance, animal studies show combining pesticides worsens the impact on thyroid hormone synthesis (193), thyroid genes (194), weight gain due to lowered thyroid hormone activity (195), thyroid gland function (196), and thyroid development (197) (198). In some cases, these effects were observed when exposure was at lower levels than what currently exists in the environment.
Unfortunately, however, few studies exist examining the effects of pesticides in combination and researchers are calling for more studies.
A Need for Improved Safety Testing of Pesticides
Clearly, we need more research and more acceptance of approaches to improve safety and better ensure thyroid health (205). This would not only better protect our health and that of our children, but also that of our pets, wildlife, and the environment (206).
In 2018, the European Union adopted criteria for identifying harmful pesticides (207) and the risks of pesticide mixtures to thyroid health are increasingly being recognized (208), but major safety gaps still exist throughout the world.
While TSH, T3, and T4 are the most common markers to measure the impact of pesticides on the thyroid, we also need to include evaluating the impact on the HPT axis given most pesticides work on the nervous system.
Although all classes of pesticides are associated with disrupting the delicate HPT axis, manufacturers are not required to research this potential action before releasing a new compound to the market.
The Takeaway When it Comes to Pesticides and Hypothyroidism
Hypothyroidism affects more than 20 million Americans, or about 12 percent of the population. Hashimoto’s is one of the most common autoimmune diseases today, affecting millions more. Given this, it should come as no surprise that thyroid hormone is one of the number one medications prescribed.
Additionally, we have been seeing a staggering rise in neurodevelopmental disorders in children and neurodegenerative disease in adults, which has drawn attention to the widespread use of pesticides in our environment (199).
Healthy brain function is required for healthy thyroid function, and numerous studies show how pesticides like glyphosate negatively impact communication between the brain and the thyroid (200) (201).
Also, it’s important to remember that the impact of pesticides on health are not going to cause immediate symptoms but instead do their work cumulatively over time. Consistent exposure to pesticides can change how the immune system functions, which may make a person more vulnerable to developing an autoimmune disease such as Hashimoto’s hypothyroidism.
What I have noticed over the years working with clients is that many times their symptoms are not resolved simply by taking thyroid medication. Many do see improvement, but others need to increase the dosage over time when the previous dose is no longer working. Still others increase the dosage and still do not see mitigation of their symptoms. I believe these pesticides interfere in thyroid hormone effectiveness and uptake at receptor sites just like they interfere in our body’s own hormones. .
Given these potential issues, it’s important to practice a detox lifestyle, reducing exposures in food and avoiding use of pesticides around the home and with pets. Most importantly, I advise adopting a detox program or protocols (like infrared saunas) that reduce the toxic load of these pesticides and chemicals in the body and fat tissues.
How to Detox and Protect Your Thyroid From Pesticides
Pesticides are ubiquitous in our environment and we need to use safe and natural tools daily to keep the thyroid detoxed and healthy.
Even though your goal may be to protect your thyroid, you need to think in terms of systemically detoxing pesticides and other environmental toxins like heavy metals from your entire body.
The goal to detox the thyroid and protect it daily from pesticides and toxins is a multi-step process:
- Taking substances like chelators that mobilize pesticides, heavy metals and toxins from body tissues like the fat, tissues, organs and glands.
- Bind the toxins that have been mobilized by supplements or infrared saunas so they don’t lodge elsewhere in the body
- Support the body’s pathways of elimination so they can exit the body (like supporting liver and lymph function).
- Mineralize the body so heavy metals and toxins don’t take the place of valuable minerals due to deficiency.
- Employ detox protocols like infrared saunas to hasten detox of body tissues and glands like the thyroid.
If you’d like to learn more about detoxing our body systemically, which will promote better thyroid hormone production, please inquire about our various other programs to detox the body of toxins like pesticides and heavy metals.










